Future of Colour
Coloured pigments play a big role in our everyday life, and that is why scientists are trying to find newer ways of making, cheaper, safer and brighter colours. This section will look at some of these colours and why they are so important to us.
Many colours, such as the traditional reds, contained mercury which is very toxic and so scientists replaced them with organic red pigments. There is one problem with these pigments in that they tend to fade in sunlight. This is obviously a problem, and so now scientists are trying to develop other red pigments that are safe but will not fade in sunlight.
In this section we are also going to look at some smart materials which are being used and developed today.
Illustrated below are the different ways in which light can interact with pigments to give an effect.
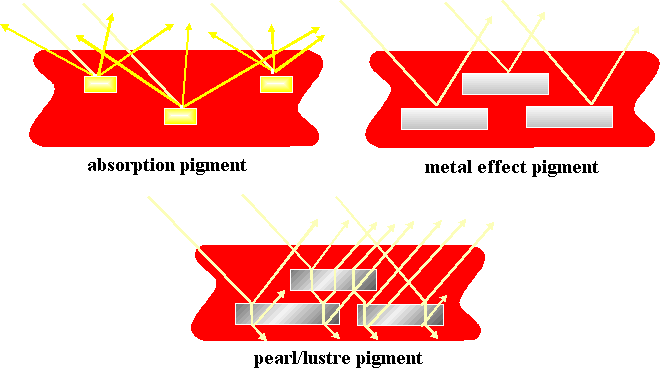
Lustre

Lustre pigments are one of the newest types of pigments available. These pigments are used in make-up, car paints, and many other items.
Lustre pigments:
- Originally influenced by their observations in nature (pearls and butterfly wings)
- Light is broken down into its components as in rainbow colours
- The different colours of light are either reflected or pass through
- As the lustre pigments are made up of layers the light gets reflected at different layers and so a glittery effect is seen.
Thermochromic

Thermochromic pigments are sophisticated pigments that can change colour when exposed to heat. This can be in the form of your body heat for many pigments or from hot water. At room temperature the structure reflects certain wavelengths of light and absorbs the others. When the pigment is heated, the molecule can gain enough energy to change its structure, and this effects the way that the lights' wavelengths are absorbed.

The heat gives the molecule enough energy to create a bond in the molecule thus changing the shape. This in turn affects the colour seen, so before you may see a nice orange pigment, once heat is supplied it is now seen to be colourless. If you mix the colours with other pigments for example a red pigments with a blue thermochromic pigment, the room temperature colour would be seen as purple. When heat is added to it the thermochromic pigment molecules change shape and appear colourless, but the red does not change so appears to the eye as if it has changed from purple to red.
Thermochromic pigments can be used to indicate temperature changes clearly. For example kettles which turn red when hot clearly indicate when it is dangerous to touch them. Babies bottles with a coating of thermochromic pigment show when the milk is too hot, and would scold the baby. Autoclaves using thermochromic pigment show a clear colour change when the sterilising agent is at the required temperature. Chemistry allows control of the temperature at which the thermochromic change occurs.
Photochromic

Photochromic pigments are types of sophisticated pigments, which can change colour due the effects of an external stimuli - in this instance UV light (photo meaning light and chromic meaning colour).
The structure initially reflects all wavelengths of light so that the pigment appears colourless. When the molecule is exposed to UV light it is supplied with sufficient energy to break a bond, allowing it to change its structure. This means that now some of the wavelengths of light can be absorbed while others are reflected, and so now it appears to possess colour when it did not before. The colour change relies on UV light from the sun or a another source such as a specialised lamp.
Ultra violet photochromics
UV pigments are very important in safety of special documents such as money, passports, prescriptions and many more. The UV pigment absorbs UV light and emits light with waves at a different frequency, these new light waves are consistent with visible light and thus appear to be a different colour - such as red, blue, green or yellow.
When the UV light source is removed the colour appears to have vanished. This is very useful as the unique blend of the pigment used on these documents is hard to replicate so people can't make their own documents, which helps reduce fraud. Continued research into new UV sensitive pigments suitable for security markings is necessary to stay one step ahead of the counterfeiters.

We can learn from nature about UV sensitive pigments. One natural material found in Greenland is hackmanite. This mineral (shown to the right) changes from colourless to purple in a few seconds, and this can be reversed by exposure to light. We have been successful in replicating the photochromic pigment in the laboratory. The composition and structure can be fine tuned to make materials that turn pink or blue when exposed to UV light. The materials are extremely stable. In addition to use for security markings these materials can be sued in sunscreens, smart windows and nail varnish.
Environmentally Friendly Red and Yellow Pigments

Ceramics, plastics and glasses are coloured using inorganic pigments which can withstand the high processing temperatures that would destroy their organic counterparts. Traditionally such inorganic pigments use heavy metals to produce vibrant colours such as red (cadmium selenide), yellow (lead chromate), purple and green. Such heavy metal pigments are damaging to the environment as they can be toxic to prepare, use and destroy.
Recently, we have been instrumental in producing some new pigments that have the bright colours and the temperature stability of the traditional inorganic pigments, but are inherently non-toxic. These materials are based on a simple niobium stannate system with small amounts of sulfur incorporated to tune the colour. The pure oxide is vibrant yellow, but by increasing the sulfur content of the mixture the colour can be tuned through orange to red. One interesting point about the pure oxide is that it is also thermochromic. Although yellow at low temperature, above 50°C it changes rapidly to bright red. Thermochromic pigments have many safety applications and are used in autoclaves, babies bottles and refrigerators.
Pigments for Reducing Overheating
Almost half the sunlight reaching the Earth’s surface is in the infra-red region, that is we cannot see it but we feel it as heat, and it contributes significantly towards the warming of the Earth’s atmosphere. The effect is most apparent in buildings and greenhouses where the IR radiation contributes to high temperatures but is of little use in, for example, photosynthesis.
Developing materials that absorb or reflect this infra-red portion of sunlight is thus a major goal as these pigments can be used in coatings and plastics to reduce the level of heating inside buildings. We are developing such materials – especially those which are colourless and do not absorb in the visible part of the spectrum. Research focuses on modifying materials which normally strongly absorb visible light and are thus intensely coloured. By altering their structures and compositions it is possible to change them so they absorb only in the near infra-red – ie just beyond visible red light. These materials are colourless allowing visible wavelengths to pass while absorbing the unwanted heat radiation. Additional applications of such materials include their incorporation into plastics for laser marking; an infra-red laser may be used to write into such transparent plastics containing the pigment as absorption of laser radiation causes the plastic to char where the laser beam is directed.
In the future we intend to develop pigments that are strongly infra-red reflecting – that is they would reflect the unwanted part of the solar spectrum back into space. Such materials may have a part to play in combating global warming.
Nanotechnology
Nanomaterials are particles so small that you need a microscope to see them (these molecules are so small that a human hair is thousands of times larger than them!) Nanopigments have special properties and are a positive example of the use of nanodots.
One feature of these molecules is that the characteristic colour properties of the molecules change with size. Quantum dots are a type of nanocrystal which is seen to change colour as they change in size. There is a lot of potential for this type of chemistry, and so research is currently being performed into this area. By varying the size of these quantum dots dramatic changes in colour can be obtained.
There are other amazing facts about these quantum dots - when they are excited with long wave ultra violet light they can emit visible light, this is a process called phosphorescence. The larger crystals will emit low energy visible wavelengths (such as orange) and the smaller crystals will emit higher energy visible wavelengths (such as blue).






