Science of Colour
The world around us would be very boring without colour. Scientists make many of the colours around you, from the paint on your walls to coloured packaging. Before scientists became involved in making coloured pigments, the range of colours was limited to natural colours that could be mined from the ground. For example, Lazurite is a blue semi-precious mineral that is difficult to extract from the ground. However, in 1828 it was synthesised synthetically in the laboratory, which made it over 1000 times cheaper. More recently, chemists develop ways also learnt to modify the colour, and produced a range of shades including purples and pinks.
How We See Colour
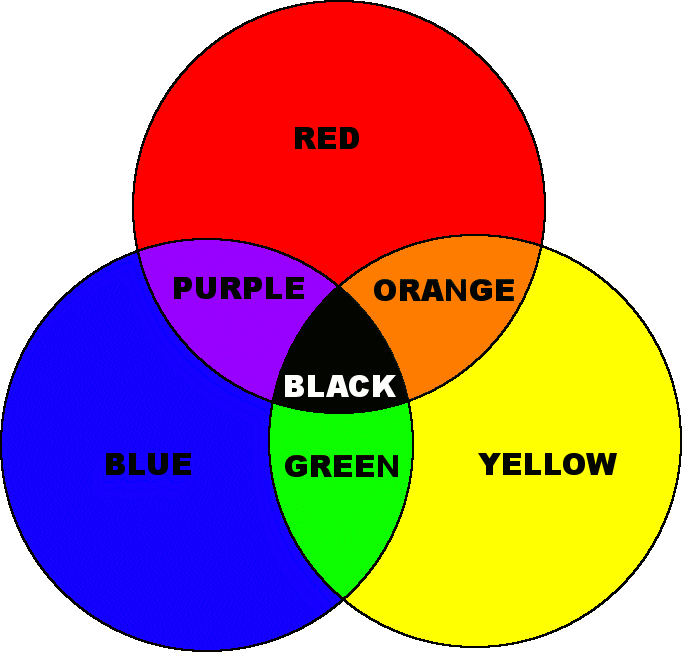
Colour comes from three main categories
- Made light - sun, light bulb, candle, light stick.
- Lost light - pigments, dyes, opals, butterfly wings.
- Moved light - rainbows, fluorescent materials.
The colour a pigment appears is the result of 'lost light.' Pigments absorb some wavelengths of light, and reflect the rest. The colour that we see is the light that is reflected back to our eyes. So when we see a red apple it is the red wavelengths that are reflected from the apple to our eyes, and the other wavelengths are absorbed by the apple. The diagram to the right illustrates how colours are formed by this 'subtractive model'. The model is named subtractive because your eye sees the remaining light after some of the spectrum has been subtracted (absorbed) by the pigment. When a pigment appears black it is because it absorbs all visible light. When all wavelengths are reflected our eyes see white.
Chemists can control the properties of the pigment so that they are not only coloured, but are stable to light, heat and other chemicals. Scientists can also make colours by using chemiluminescence, light emitting diodes (LED) and phosphors.
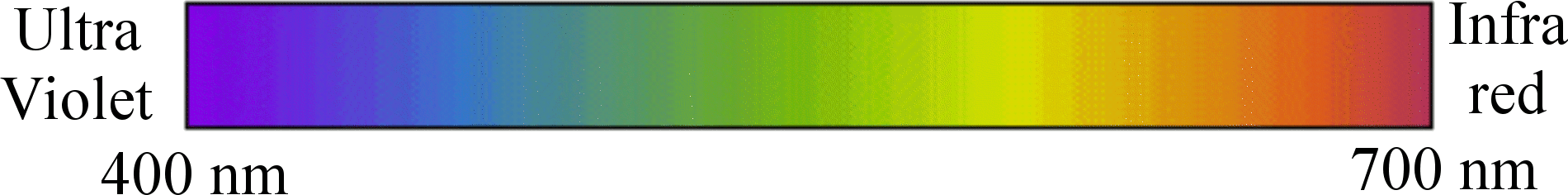
Colour in the Electromagnetic Spectrum
Sunlight is made up of a range of wavelengths of the electromagnetic spectrum. Visible light has a wavelength of between 400-700 nanometres (1 nanometre = 1 billionth of a metre). Purple has the shortest wavelength, and red has the longest wavelength. Above 700 nanometres is the infra red region of the spectrum, which we feel as heat. Below 400 nanometres is the ultra violet region of the spectrum, which has more energy and causes sun burn. New smart pigments can react to these other parts of the solar spectrum to create intensity and useful effects (e.g. heat shields or security markers.
The Eye
The eye is an organ of the body which allows us to see. The retina is the part of the eye that detects light, and is made from rods and cones. Rods detect black and white, and are more sensitive than the cones. That is why in dim light you can only see black and white. The cones detect colour.






