Making Pigments
There are two main methods for preparing pigments in the laboratory. An example of each method is given in the experiments below.
Solid State Synthesis
Solid state synthesis involves mixing two solid starting materials together, and then heating the mixture to high temperatures to form the pigment. Initially it is necessary to weigh out the starting solids.

After weighing, the starting materials are mixed and ground in a pestle and mortar. They are then placed in a crucible and heated to high temperature. We have made pigments at temperatures from 500°C to 1200°C. Below is a picture of a sample being placed into a furnace.

Once the solid pigment has been made in the furnace it is allowed to cool to room temperature

After the material has been cooled, it is characterised to make sure we have formed the pigment we want. We do this using X-ray crystallography. Below is a picture of some pigments in an X-ray crystallography machine.
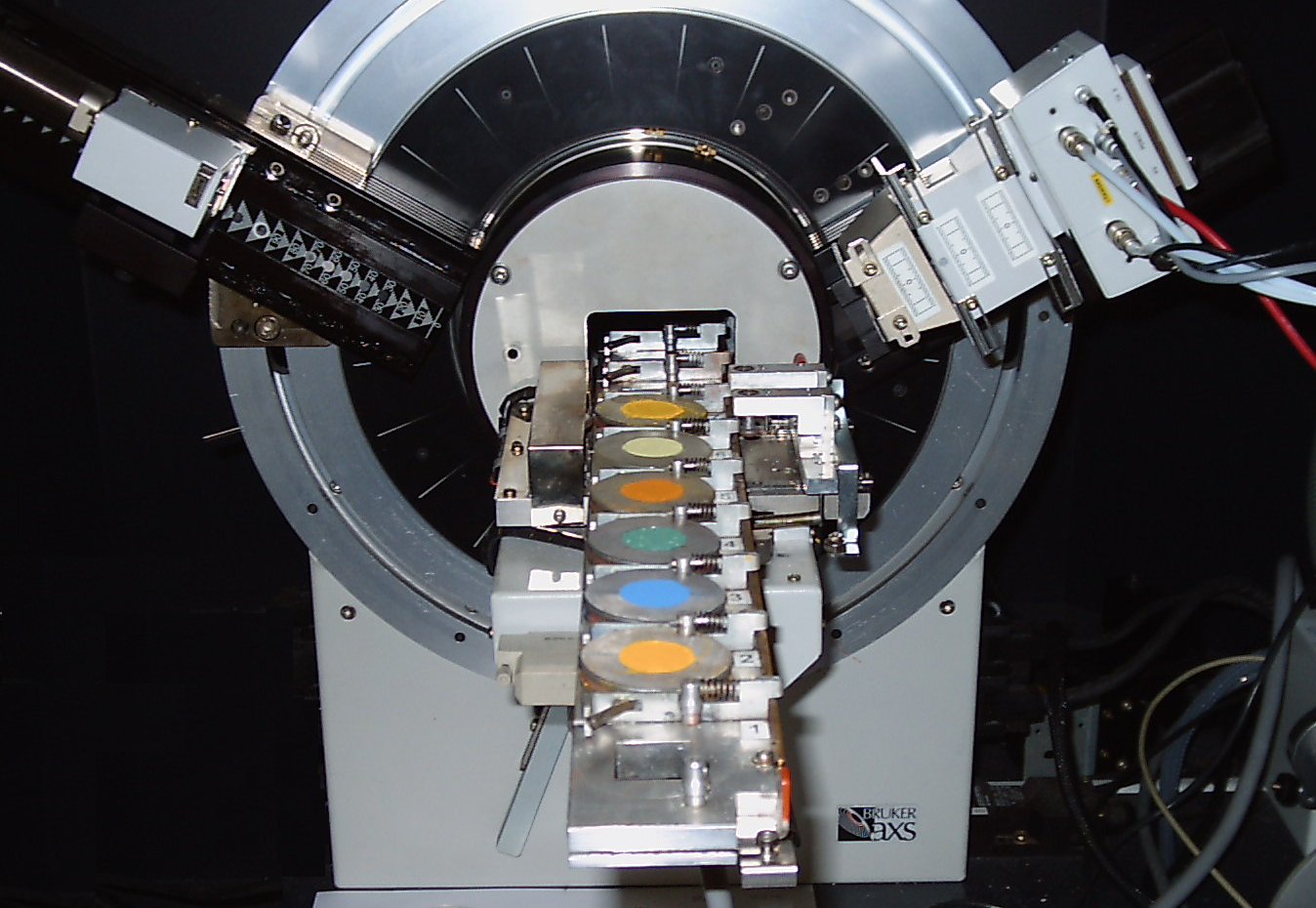
Precipitation
Precipitation is a method for preparing pigments where two solutions are mixed together and a solid is formed. A solid is formed because when the solutions are mixed a chemical reaction takes place, which forms an insoluble pigment. Initially it is necessary to prepare the solutions containing the starting materials. This is done by weighing a mass of solid and dissolving it in an appropriate amount of water, usually using a volumetric flask.
Once the solutions are prepared, then they are transferred to a beaker and a volumetric flask.

The solutions are then mixed, and a precipitate is formed.

The precipitate is then filtered off, before it is dried.

Finally the pigment is collected. The example shown in these pictures is the formation of Prussian Blue, from ferric chloride and potassium ferrocyanide.







